The Basics of Graft-Versus-Host Disease
Graft-versus-host disease (GvHD) is a potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation and a major cause of morbidity and mortality post-transplant. GvHD occurs when the donor T cells mount an immune response against the recipient’s organs and tissues.
The risk of GvHD may be influenced by the graft source (bone marrow vs. peripheral blood), age of the recipient, and degree of genetic similarity between the donor and recipient [1]. GvHD is typically categorized as acute and/or chronic. Symptoms in both acute and chronic GvHD range from mild to life-threatening.
Acute GvHD
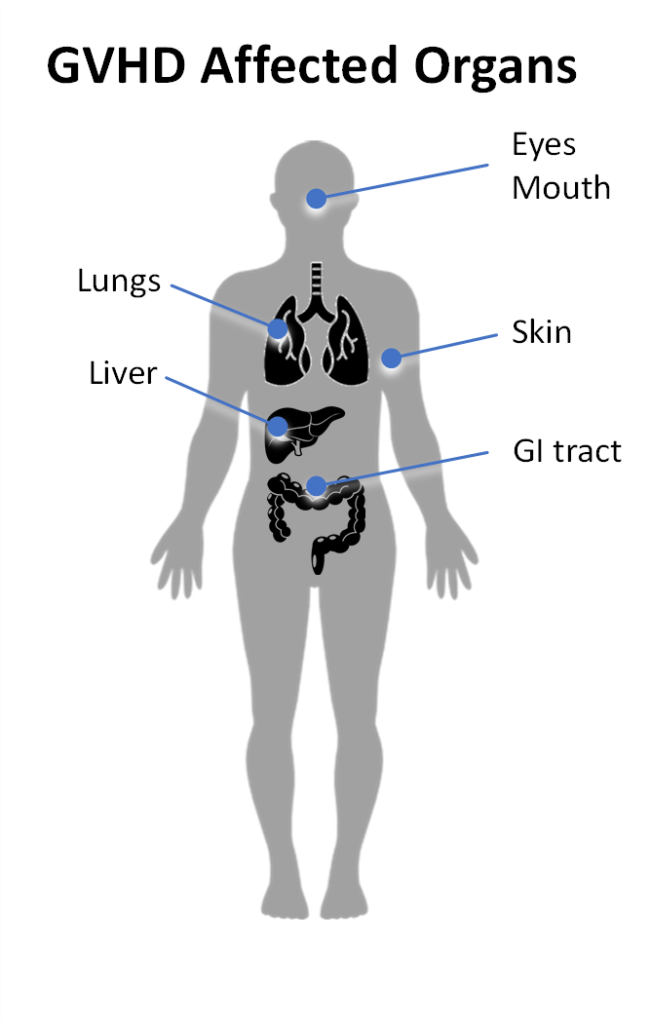
Acute GvHD can occur at any time, but often occurs in the first 100 days after transplant [2]. Acute GvHD is characterized by an inflammatory response driven by alloreactive donor T cells. These T cells attack the patient’s skin, liver and/or the GI tract. Typical symptoms of acute GvHD include:
- Blisters on the skin or rashes
- Persistent nausea
- Diarrhea
- Blood in stool
- Jaundice
Chronic GvHD
Chronic GvHD can occur at any time following transplantation, but typically manifests several months after the transplant. Chronic GvHD is characterized by an alloimmune response that may also involve an autoimmune component including autoantibody production [3]. Chronic GVHD symptoms may include:
- Dry eyes
- Mouth sores
- Fatigue or muscle weakness
- Joint pain or stiffness
- Skin rash or loss of pigmentation in the skin
- Skin texture changes
- Persistent cough
- Nausea or vomiting
- Diarrhea
- Weight loss
The standard of care for initial GvHD therapy is systemic corticosteroid. Unfortunately, approximately 50% of patients with GvHD will not achieve an adequate response to steroids. Many of these patients may become steroid-refractory and require additional immunosuppressive therapy. Many of these therapies have significant side effects, including kidney and liver damage.
TCR Biotherapeutics was founded to solve the unmet need for a highly effective GvHD therapy with mild or no side effects. Our initial product candidate, TCR001, is currently in preclinical development ahead of first-in-human clinical trials. Follow us on social media for updates (click icons below) or contact us to learn more.
References: [1] Arai, et al. Biol Blood Marrow Transplant. 2015 Feb; 21(2): 266–274. [2] Nassereddine, et al. Anticancer Res. 2017 Apr;37(4):1547-1555. doi: 10.21873/anticanres.11483. [3] Lee, SJ et al., Biol Blood Marrow Transplant. 2003 Apr; 9(4): 215-33. doi: 10.1053/bbmt.2003.50026.

Comments are closed